
BriaCell’s Patented Immunotherapy
Bria-IMT™
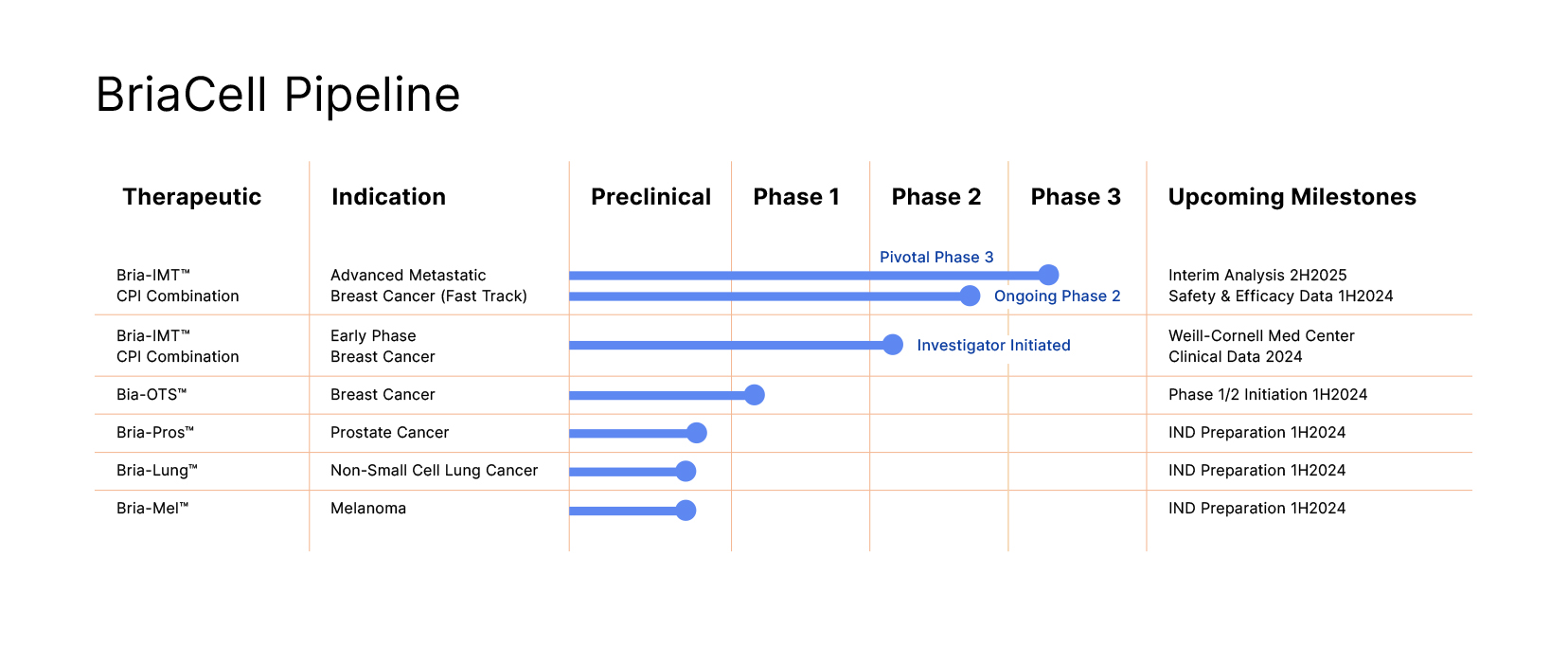
Bria-IMT™, our lead drug candidate currently in Phase 3, is a patented, off-the-shelf, cell based, targeted immunotherapy that stimulates the patient’s immune system to induce targeted killing of cancer cells.
Our data shows that Bria-IMT™, an off-the-shelf immunotherapy, expresses tumor antigens and granulocyte-macrophage colony-stimulating factor (GM-CSF) to activate specific cancer fighting CD4+ and CD8+ T cells, and stimulates the immune system to enhance targeted killing of cancer cells. When combined with a check point inhibitor, which “releases the brakes”, Bria-IMT™, “steps on the gas” providing powerful anti-tumor activity.
BriaCell recently announced initiation of a pivotal Phase 3 study in advanced metastatic breast cancer, which if successful could lead to full approval of Bria-IMT™ in combination with a check point inhibitor. BriaCell also announced completion of enrollment of advanced breast cancer patients in its randomized ongoing Phase 2 combination study of Bria-IMT™ with Incyte’s immune checkpoint inhibitor, retifanlimab. This study followed successful completion of a Phase 1 study showing a favorable safety and efficacy profile for the combination regimen. Data includes unprecedented activity in very advanced patients who have failed antibody-drug conjugate therapy and in patients with metastases to the central nervous system.
Off-The-Shelf Personalized Immunotherapy
Bria-OTS™
Our next generation personalized off-the-shelf immunotherapy, Bria-OTS™, Our next generation personalized off-the-shelf immunotherapy, Bria-OTS™, awarded numerous US and international patents and supported by clinical data, forms the basis of BriaCell’s “OTS” strategy.
Introducing Bria-OTS™
- Bria-IMT™ is most effective in HLA – type matched patients
- Bria-OTS™ is engineered to express 15 unique HLA types in 4 independent cell lines
- Provides matched treatment to greater than 99% of patients with advanced breast cancer
- Simple saliva test determines patient HLA type for delivery of personalized off-the-shelf Bria-OTS™ immunotherapy
- Received a Small Business Innovation Research (SBIR) award from the National Cancer Institute (NCI)
- Ongoing collaboration with the NCI
- Similar cell lines in development for prostate cancer (Bria-Pros™), lung cancer (Bria-Lung™), and melanoma (Bria-Me™) including enhanced ability to activate resting (naïve) T cells



BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW; TSX: BCT) is a clinical stage immuno-oncology company that is developing an entirely new class of targeted cellular immunotherapies to transform cancer care
- Our lead drug candidate is Bria-IMT™. Developed by Dr. Charles Wiseman, and Dr. Alex Kharazi, Bria-IMT™ was designed to treat advanced metastatic breast cancer patients (i.e. patients in whom the cancer cells have spread beyond the breast) who did not respond to chemotherapies.
- According to American Cancer Society Facts and Figures, breast cancer is the most common cancer in the United States with 313,510 expected new cases in 2024.
- 42,780 women and men are expected to die of breast cancer in the U.S. in 2024, making breast cancer the 2nd leading cause of cancer death in women in the U.S.
- Immune Check Point Inhibitors (CPIs) work by neutralizing immune suppression that may be blocking the killing of cancer cells.
- Combining Bria-IMT™ with a CPI may significantly increase a patient’s immune system cancer fighting ability.
- Incyte (Nasdaq: INCY) → BriaCell has established a corporate collaboration and supply agreement with Incyte.
- Currently evaluating Bria-IMT™ in a pivotal Phase 3 combination study with an immune check point inhibitor.
- Bria-OTS™: BriaCell is developing a personalized “Off-The-Shelf” cellular immunotherapy based on a patient’s HLA-type.
- Experienced Management Team and Board of Directors; Clinical Strategy Team involved in 19 drug approvals.

