Personalized Off-The-Shelf Immunotherapy
Bria-OTS™: Personalized Off-the-Shelf Immunotherapy
BriaCell Phase 1/2 Study of Bria-OTS™, also known as Bria-BRES™, in metastatic breast cancer is open
The Phase 1/2 clinical study is listed on ClinicalTrials.gov as NCT06471673.
• Bucket trial with additional cancer indications planned
• Enhanced version (Bria-OTS+™) scheduled to enter the clinic 1H2025 starting with Bria-Pros™ (prostate cancer)
==========================================================================================================================
• We believe Bria-IMT™ is most effective in human leukocyte antigens (HLA) – type matched patients
• Bria-OTS™ is engineered to express 15 unique HLA types through 4 independent cell lines
• Provides matched treatment to greater than 99% of patients
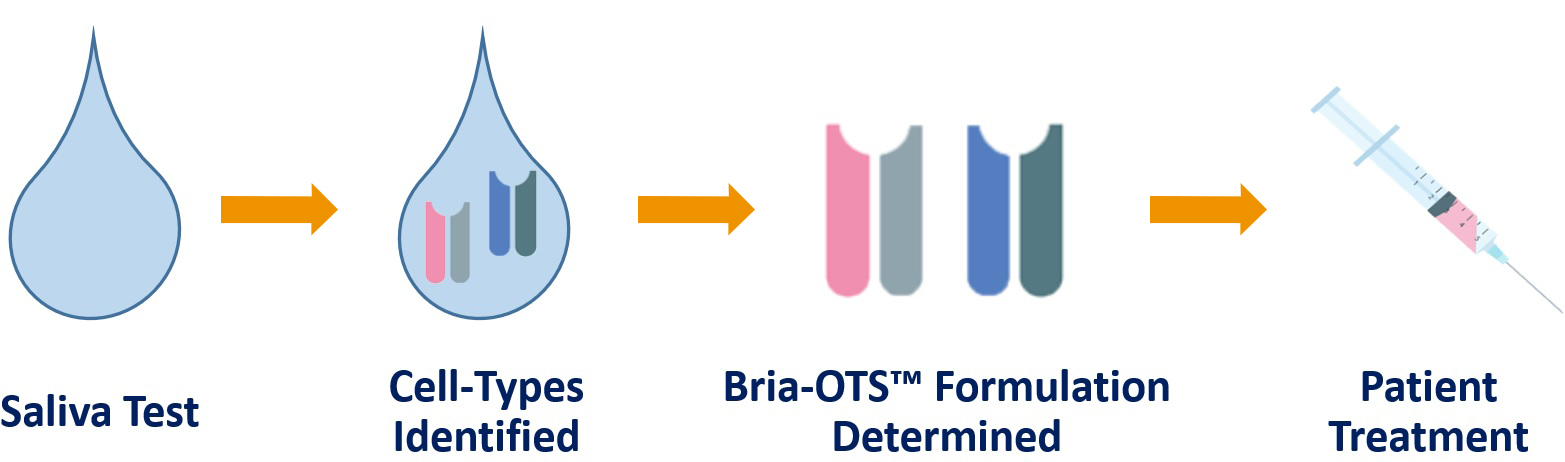
• Simple saliva test provides HLA matched personalized off-the-shelf Bria-OTS™ immunotherapy
• HLA matched off-the-shelf therapy is faster and less costly than other expensive and complex personalized immunotherapies
• BriaCell received a Small Business Innovation Research (SBIR) grant from the National Cancer Institute (NCI) to further develop Bria-OTS™
• Ongoing collaboration with the NCI to investigate the Bria-OTS™ mechanism action
• BriaCell has secured numerous US and international patents for Bria-OTS™
• Similar immunotherapies are in development for prostate cancer (Bria-Pros™), lung cancer (Bria-Lung™), and melanoma (Bria-Mel™)
What Is Personalized Cancer Immunotherapy?
Personalized cancer immunotherapy refers to cancer immunotherapy treatments tailored to each patient’s unique tumor and immune profile. This approach falls under the broader field of precision oncology and aims to create a customized immunotherapy plan designed for the individual rather than a one-size-fits-all treatment.
Traditional personalized cancer treatment often involves complex, labor-intensive steps. These may include harvesting a patient’s immune cells, modifying them in a laboratory setting, and reinfusing them into the body. While effective, these methods are time-consuming and costly, and they’re not always feasible for patients with aggressive or advanced disease.
BriaCell’s approach with Bria-OTS™ represents a major shift in how we think about personalization. Instead of manipulating a patient’s own cells, Bria-OTS™ uses premanufactured cells that are matched to the patient’s HLA type, a key component of the immune system responsible for identifying threats and starting an immune response. By using HLA matching as the personalization engine, Bria-OTS™ delivers a tailored immune response without the delays and complications of traditional methods.
This off-the-shelf strategy makes customized immunotherapy more accessible and scalable while still maintaining the benefits of precision targeting.
Why Personalized Cancer Immunotherapy Matters
The success of cancer immunotherapy depends on how well we can train the immune system to distinguish cancer cells from healthy tissue. Personalized cancer treatment allows for tumor-specific targeting, which means better outcomes and fewer side effects for patients.
Here’s what makes personalized cancer immunotherapy so important:
- Improved targeting. Because treatment is based on tumor antigens unique to each patient’s cancer, the immune system can precisely locate and attack the malignancy.
- Reduced toxicity. With fewer healthy cells affected, patients often experience fewer side effects compared to traditional treatments, such as chemotherapy or radiation.
- Adaptive response. The immune system can evolve its response to recognize and address cancer mutations over time, offering ongoing protection.
- Broad applicability. This method holds potential across many cancer types, especially those resistant to existing therapies.
Matching a patient’s HLA type assures that tumor antigens elicit immune activation which ensures the treatment engages the right mechanisms to destroy tumor cells. This tailored immune response increases efficacy, improves durability, and reduces the risk of the immune system attacking healthy cells — a problem known as off-target toxicity.
Personalization also plays a critical role in overcoming resistance to certain therapies. Some tumors evade treatment by mutating or suppressing immune responses. Personalized immunotherapy can adapt to these changes by targeting tumor-specific markers and bypassing resistance pathways, including those seen with checkpoint inhibitors and antibody-drug conjugates.
Checkpoint inhibitors, while powerful, don’t work for every patient. By customizing the immune response, we can improve the chances of success even in resistant types of cancer. For many patients with advanced disease, this personalized approach can make the difference between short-term remission and long-term survival.
BriaCell remains committed to making cancer immunotherapy more precise, effective, and accessible. By focusing on the immune system and using technologies such as HLA-matching, we believe in the power of a smarter, personalized fight against cancer.

